Within the cannabis plantlays a wide range of different chemical compounds, including terpenes and cannabinoids. Of the cannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD) and the most well known and well studied. And while millions use these two medicinally and recreationally every day, you may be surprised to learn that these compounds must undergo a chemical reaction before they can take their effects on the body and brain [1].
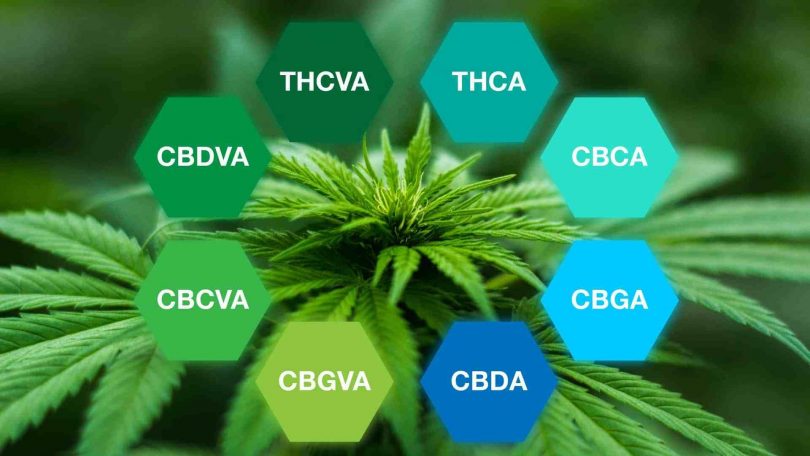
Raw cannabis, which consists of the iconic flowers, trichromes, and green-to-purple hues, contains cannabinoid acids. These acids are converted into the types of cannabinoids you are most familiar with through a series of biosynthetic steps.
This process begins with cannabigerolic acid (CBGA) and cannabigerovarinic acid (CBGVA), which have been called the “mother” cannabinoids [2]. CBGA and CBGVAare formed through the synthesis of phytochemicals. Depending of the type of enzyme (THCA/CBDA synthase),these chemical precursors can be transformed into tetrahydrocannabinolic-acid (THCA) and cannabidiolic acid (CBDA), as well as several other acids. From there, through a process called decarboxylation (loss of carbon dioxide), THC and CBD are born.
Heat and UV light can be used to decarboxylate THCA and CBDA [3]. The most common way to achieve this transformation is by lighting up the cannabis plant, a method that has been used for many, many years. Of course, if you are consuming a cannabis concentrate, all of this processing has already occurred before you’ve purchased your product.
One of the most important distinctions between non-activated cannabinoid and activated cannabinoids is their effects – while THC is psychoactive, THCA (and the other acids) are not. But these acids also contain a host of benefits, some of which are similar to that of CBD and THC and some of which are unique [4].
Preliminary studies on THCA have shown that this acid may be an effective anti-inflammatory, antiemetic, and neuroprotective agent; in fact, THCA may be a promising novel compound to treat Huntington’s disease [5-7]. Research on tetrahydrocannabivarin (THCV) has shown that it may be effective as an anti-obesity treatment, since, in stark contrast to THC, THCV appears to actually suppress appetite [8].
Cannabidiolic acid (CBDA) may have therapeutic potential in preventing the spread of breast cancer [9]. Additionally, cannabichromenenic acid (CBCA) may possess antifungal and antibacterial properties [10].
We have a long way to go to better understand the potential of these non-activated compounds. For so many years, we heated cannabis to inhale its vapors or create concentrates without even considering its properties in raw form – but, now with renewed interest and advancements in cannabis science, we certainly will not let these the potential of these unique properties go up in smoke.
References
- Russo, E.B, “Taming THC: Potential Cannabis Synergy and Phytocannabinoid-terpenoid Entourage Effects”, Br J Pharmacol, 2011, Volume 163, pg. 1344-1364.
- Thomas, B.F., ElSohly, M.A., “Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations”, Chapter 2 – Biosynthesis and Pharmacology of Phytocannabinoids and Related Chemical Constituents, The Analytical Chemistry of Cannabis, 2016, pg. 27-41.
- Lewis, M.M., Yang, Y., Wasilewski, E., Clarke, H.A., Kotra, L.P., “Chemical Profiling of Medical Cannabis Extracts”,ACS Omega, 2017, Volume 2, pg. 6091-6103.
- Izzo, A.A., Borrelli, F., Capasso, R., Di Marzo, V., Mechoulam, R., “Non-psychotropic Plant Cannabinoids: New Therapeutic Opportunities from an Ancient Herb”, Trends Pharmacol Sci, 2009, Volume 30, pg. 515-527.
- Nallathambi, R., Mazuz, M., Ion, A., et al., “Anti-Inflammatory Activity in Colon Models Is Derived from Δ9-Tetrahydrocannabinolic Acid That Interacts with Additional Compounds in Cannabis Extracts”, Cannabis Cannabinoid Res, 2017, Volume 2, pg. 167-182.
- Rock, E.M., Kopstick, R.L., Limebeer, C.L., Parker, L.A., “Tetrahydrocannabinolic Acid Reduces Nausea-induced Conditioned Gaping in Rats and Vomiting in Suncus murinus”, Br J Pharmacol,2013, Volume 170, pg. 641-648.
- Nadal, X., Del Río, C., Casano S., et al., “Tetrahydrocannabinolic Acid is a Potent PPARγ Agonist with Neuroprotective Activity”, Br J Pharmacol,2017, Volume 174, pg. 4263-4276.
- Greenway, F.L., Kirwan, J.P., “Medical Marijuana—An Obesity Problem or Opportunity?”, International Journal of Obesity, 2019, pg. 1-2.
- Takeda, S., et al.,“Cannabidiolic Acid-mediated Selective Down-regulation of c-fos in Highly Aggressive Breast Cancer MDA-MB-231 Cells: Possible Involvement of its Down-regulation in the Abrogation of Aggressiveness”, J Nat Med, 2017, Volume 71, pg. 286-291
- Turner, C.E., Elsohly, M.A., “Biological Activity of Cannabichromene, its Homologs and Isomers”, J Clin Pharmacol, 1981,Volume 21, pg. 283S-291S.